Background: Risk stratification for pediatric AML currently relies on response to therapy and a small number of prognostic genetic factors. With these limited criteria, approximately 75% of children are initially classified as being at a lower risk (LR) of relapse. However, 30% of LR patients relapse, and achieving a second remission is often quite difficult. Therefore, there is an urgent need improve risk stratification in pediatric AML. Cytokine levels have been shown to predict outcome in adult AML but have not been extensively studied in pediatric AML, in part due to lack of available samples. We utilized a unique repository of diagnostic peripheral blood (PB) plasma from pediatric AML patients to quantify cytokine levels and determine associations with outcome.
Methods: Peripheral blood plasma samples from pediatric AML patients enrolled on the multi-institutional Children's Oncology Group (COG) protocol AAML1031 were studied. We obtained diagnostic PB plasma for 80 patients initially classified as LR. Samples were collected into Cellsave® tubes, which are designed to preserve tumor cells and cytokines during transport. For comparison, we obtained normal PB (NPB) plasma from 11 healthy siblings undergoing blood draws for HLA typing. Cytokine levels were determined with the 21-plex Milliplex High Sensitivity T-cell Panel and the Human Myokine Panel (EMD Millipore). Cytokine and growth factor concentrations between NPB and AML PB plasma samples were analyzed by the Mann-Whitney U test, and correlation of cytokine levels with clinical outcome was assessed by the Kaplan-Meier method.
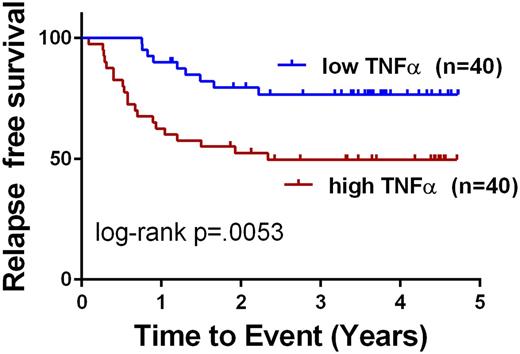
Results: In this cohort of samples we found the following cytokines and growth factors to be significantly higher in AML patients v. controls: IL-6, IL-10, IL-17A, TNFα, IL-2, IL-1β, IL-12(p70), MIP-3a, and Fractalkine. Interestingly, and discrepant from what has been described previously in adult and pediatric studies, IL-8 levels were not significantly different when compared to normal controls in this cohort. When corrected for multiple comparisons by the Bonferroni method, IL-6, TNFα, MIP-3a, and IL-1β remained significantly different. For IL-6, the median in NPB was 2.8 pg/mL (25-75 Percentile 1.43-6.3) vs 12.31 pg/mL for AML (25-75 Percentile 7.29-21.09, p<.0001). For TNFα, the median in NPB was 12.1 pg/mL (25-75 Percentile 6.5-18.2) vs 21.7 pg/mL for AML (25-75 Percentile 14.9-34.9, p<.0001). For IL-10, the median in NPB was 23.9 pg/mL (25-75 Percentile 14.4-67.8) vs 64.0 pg/mL for AML (25-75 Percentile 38.9-90.9, p=.007). To determine whether elevated cytokine levels at diagnosis were associated with an increased risk of relapse, we performed cut-point analysis and evaluated outcomes for all patients. Among the cytokines that were found to be elevated in diagnostic samples, TNFα and IL-10 were associated with clinical outcome. Patients with high TNFα (n=40) had 3 year relapse free survival (RFS) of 49.6% compared to 76.6% for those with low TNFα (n=40, cut at the median 21.7 pg/mL, p=.0053, log-rank test). Patients with high IL-10 (n=11) had 3 year RFS of 36.4% compared to 67.3% for those with low IL-10 (n=69, cut-point of 112 pg/mL, p=.005, log-rank test). These differences in outcomes remained significant after Bonferroni correction. Of note, our previous study of bone marrow plasma showed that elevated IL-6 levels at diagnosis are associated with inferior RFS (Stevens et al, Blood Advances, In Press); however, in this PB study, IL-6 levels were not associated with RFS.
Conclusions: Our data demonstrate that in pediatric AML patients, IL-6, IL-10, and TNFα levels in diagnostic PB plasma are elevated in a subset of patients. Further, higher levels of TNFα and lower levels of IL-10were associated with lower RFS. This study, together with our previous finding that higher bone marrow IL-6 levels are associated with poor outcome, supports the idea that an abnormal pro-inflammatory state may contribute to poor outcomes in otherwise LR patients. Additional studies are needed to determine the mechanisms by which these cytokines reduce survival, and to further evaluate their use as prognostic biomarkers in pediatric AML patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.